Your shopping cart is empty!
MENU
- +
-
Categories+
- Blog +
- Contact Us +
- Login +
- Register +
An in-vitro culture of cell lines is a close attempt to mimic the physiological conditions and develop an understanding of normal responses and functions at the cellular levels. Generally, two types of cultured cells are used: Monolayer cultures where the cells have the ability to attach with the substrate (matrix-coated dishes, T-flasks, multiple-well plates) and Suspension cultures where the cells grow free floating in a suitable growth media.
The growth of adherent cell lines (monolayer cultures) depends on the availability of surface area and the nutrients content in the medium. As the cells reach confluency or the medium is deficient in nutrients, the cells should be subcultured or preserved so that the cell viability remains unaffected.
Cell preservation is an important step of animal cell culture and its maintenance, which is different from preserving bacteria and fungi. The most effective method for animal cell culture preservation is cryopreservation accomplished by either a suitable cryogenic agent or liquid nitrogen. The maintenance of cell lines is difficult owing to the slow growth rate and low survival rates. Therefore, an appropriate cryopreservation method is essential that can establish the high viability of the cells (approximately more than 90%). Briefly, cryopreservation involves a slow reduction of temperature of the cells to -30 to -60°C followed by flash freezing to less than -130°C. In principle, as the cells reach the ultralow temperatures, the degrading enzymes (proteases and phosphatases) become ineffective leading to a state of biological inertness. There are multiple factors that importantly affect the viability of the preserved cell lines:
a) Growth conditions at the time of cell recovery
b) Physiological condition of the preserved cells
c) Cell density at the time of preservation
d) Type of cryoprotectant
e) Freezing technique
The ideal time point of cell preservation is late-logarithmic to early-stationary phase that provides highest possible viable cells. The cells should be at the higher densities, approximately 106 to 107 cells/ml.
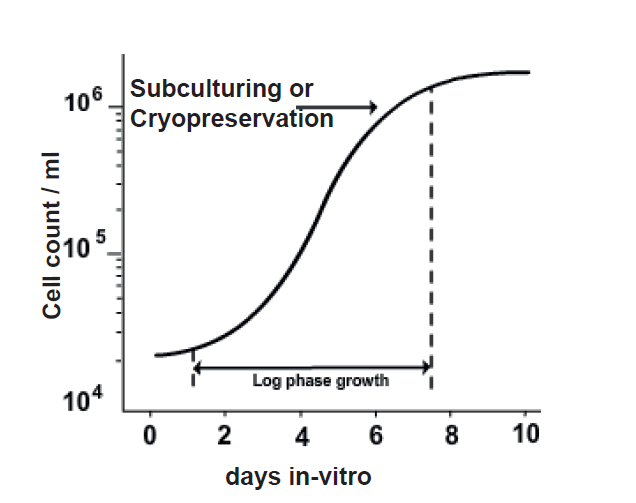
Figure: Phases for subculture or cryopreservation (modified from Freshney RI, 2010)
The choice of an appropriate cryoprotectant is crucial as well. The cryoprotective agents such as glycerol or dimethylsulfoxide (DMSO) diffuse into the cell and help to prevent dehydration during freezing by partial replacement of intracellular water. The final concentration of DMSO between 7% and 10% is the most common choice as a cryoprotectant. Glycerol is used as a cryoprotectant when the strong bi-polar compounds such as DMSO has an adverse effect on the cells, for e.g. HL-60 cells differentiate upon exposure to DMSO which might negatively affect subcultures. The Cryopreservation medium should always be the same as that of the medium used for propagation of the culture with additional Fetal bovine serum (maximum 20%). Serum proteins in the FBS bind with the toxins released from the lysed cells during the freezing or thawing process. The thumb rule to follow in cryopreservation and subsequent recovery is slow freezing and rapid thawing.
The cell freezing system from BT lab systems harnesses the slow and constant rate freezing principle which is ideal for the cryopreservation of most of the mammalian cultured cell lines and efficient in maintaining high cell viability. The system is user-friendly and based on a heat transfer core to maintain a low thermal mass. The system provides a constant freezing rate of -1 degree per minute; hence the freezing profiles do not vary at different cycles. Moreover, the system has low thermal mass, hence, there is no rise in local freezer temperature, subsequently, avoid heat dissipation from new samples to the previously stored samples.
How to operate Cell Freezing System for optimal cryopreservation of cells:
1. The instrument has 12 chambers consisting of wells to hold the tubes. Before starting the freezing process, the chambers and the tubes should be completely dry. The metal core ring should be placed in the central cavity and bring at room temperature.
2. To begin the freezing, the cryovials (2ml capacity) containing cell suspension should be placed in the slots. It must be checked that the tubes can move in and out freely without any obstruction.
3. The instrument lid should be tightened and placed in a -80°C freezer upright keeping at least 1 inch of free space between the instrument and the freezer. The instrument will freeze the cells with the rate of -1°C per minute when placed in a -80°C environment.
4. The cryovials containing cell suspension should be frozen for 4 hours before flash freezing to ultra-low temperature.
5. In order to transfer the cryovials to liquid nitrogen (Flash freezing and long-term storage), the instrument should be removed from the freezer and the frozen cryovials should be recovered by inverting the instrument over a dry ice containing insulated container. In case any vials get stuck in the slots, the inverted instrument can be tapped on a flat surface.
6. The vials can then be transferred to liquid nitrogen chambers for prolong stock preservation.
7. The viability of all the frozen cultures should be checked before using it for culturing.
Tips to consider:
References:
Coriell, L.L. 1979. Preservation, storage, and shipment. Methods in Enzymology. vol. 58:29-36.
Day, J.G. & Stacey, G.N. 2007. Cryopreservation and Freeze-Drying Protocols. Humana Press. 2nd edn.
Freshney, R.I. 2010. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications. John Wiley & Sons, Inc. 6th edn. 10.1002/9780470649367.
Morris, C.B. 1995. Cryopreservation of animal and human cell lines, Methods in Molecular Biology, Vol. 38:179-187.
Morris, C.B.M. 2000. Routine Subculturing. In Cell and Tissue Culture for Medical Research. Chapter 1.6. John Wiley & Sons, Inc. 38–44.
Leave a Comment